Understanding Keto Enol Tautomerism
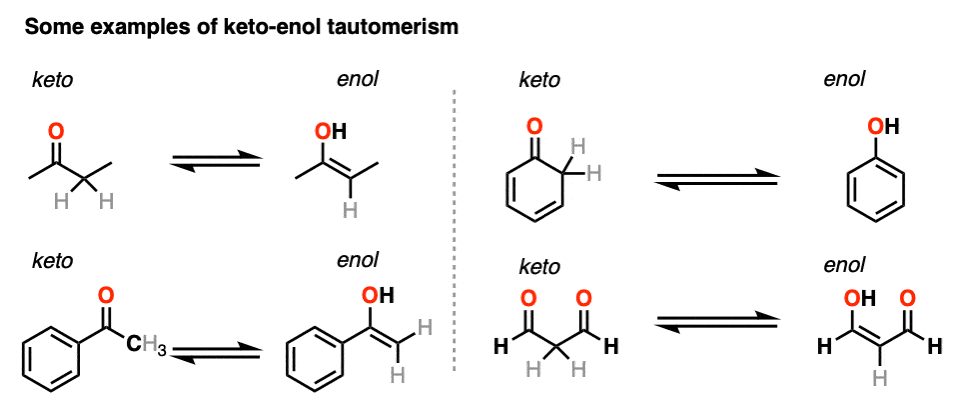
Keto enol tautomerism is a fascinating concept in organic chemistry that revolves around the dynamic equilibrium between keto and enol forms of organic compounds. This phenomenon is not only essential to understanding the stability of different isomers but also crucial in reaction mechanisms, particularly in acid-base reactions. In this comprehensive guide, we will explore the principles of keto enol tautomerism, its implications in chemical reactions, and its relevance in various applications within organic chemistry as of 2025.
The Basics of Tautomerism
Tautomerism describes the chemical equilibrium between two structural isomers that can readily interconvert, as seen in keto and enol forms. The dynamic nature of **keto-enol tautomerism** is primarily influenced by acid-base reactions and the stability of the resulting isomers. Understanding tautomeric systems entails a grasp of fundamental principles such as molecular structure, proton transfer, and acid-base chemistry. Different organic compounds may exhibit varying degrees of tautomerism based on their chemical properties.
Keto and Enol Forms
The *keto form* of a compound typically features a carbonyl group (C=O), while the *enol form* contains an alcohol functionality (C=C-OH). The stability of these forms depends significantly on **thermodynamic stability**, which can vary based on several factors, including solvent effects and hydrogen bonding. In many cases, the keto form is favored due to greater resonance stabilization; however, certain conditions may enhance the stability and reactivity of the enol form, leading to contrasting **reactivity patterns** in different reactions.
Chemical Equilibrium and Reaction Mechanisms
The transition between keto and enol forms exemplifies the importance of **chemical equilibrium** in understanding reaction mechanisms. The shift between these tautomers often involves proton transfer and is influenced by reaction conditions such as pH and temperature. For example, lower pH might stabilize the enol form due to the presence of more **acidic hydrogen** ions, facilitating the **keto-enol interconversion**. Identifying the pathways for these equilibria requires a comprehensive understanding of underlying chemical mechanisms.
Identifying Structural Isomers
Structural isomers demonstrate varying chemical properties despite having the same molecular formula. In keto-enol tautomerism, distinguishing between the structural features of the keto and enol forms is critical for evaluating their stability. Tools like *NMR analysis*, *IR spectroscopy*, and *structural formulas* play a significant role in elucidating the molecular architecture of these tautomers. By analyzing chemical transformations and the dynamic behavior of these structural isomers, chemists continue to enhance their understanding of reaction pathways and product formation.
Factors Affecting Keto-Enol Equilibrium
The equilibrium and stability of keto and enol forms can be greatly influenced by numerous factors, ranging from solvation effects to the presence of substituents. Understanding these factors is crucial for predicting the favored tautomer and the overall outcome of organic reactions.
Solvent Effects on Stability
The choice of solvent plays an integral role in determining the relative stability of keto and enol forms. Polar solvents, for instance, can enhance the solvation of the enol form, making it more stable by stabilizing the hydrogen bonding interactions within the compound. This influence of solvent polarity on **reaction rates** and *thermodynamic stability* can redirect pathways for potential reactions in organic chemistry, shedding light on solvent implications in various synthesis techniques.
Electronic Effects and Substituent Impact
Substituents on the molecular structure also recursively affect the keto-enol tautomerism. Groups that can stabilize a negative charge, such as electron-withdrawing substituents, can facilitate the formation of enol forms. Consider, for instance, a carbonyl compound where the substituents at the allylic position enhance acidity. Recognizing the importance of these electronic effects can lead to better predictions of reactivity and selectivity in chemical transformations.
Influence of Temperature and Reaction Conditions
Temperature plays a critical role in shifting equilibrium between keto and enol forms. Generally, higher temperatures tend to favor the formation of tautomers that are thermodynamically less stable. The **temperature**-to-stability relationship can optimize yields in synthetic applications where specific tautomer formations are desired. Consequently, adjusting the conditions affecting equilibrium can significantly impact the outcome of target synthesis efforts in **organic reactions**.
Applications of Keto Enol Tautomerism in Organic Chemistry
Keto enol tautomerism is a cornerstone of many synthetic applications within organic chemistry. The reactivity and selectivity offered by enol forms often enhance the versatility of synthesis and contribute to the formation of complex organic structures.
Synthetic Pathways and Tautomerism Importance
Strategies exploiting keto-enol tautomerism enable chemists to access *synthetic pathways* leading to complex compounds. For example, the enol forms often serve as nucleophiles in various **nucleophilic substitution** or addition reactions. Utilizing the inherent reactivity of the enols in combination with modern reagent methods yields advancements in target compound synthesis. This fundamental understanding highlights the significance of tautomerism in crafting new organic compounds.
Impact on Medicinal Chemistry
In medicinal chemistry, controlling the tautomeric forms of compounds can significantly affect drug efficacy and biological interactions. Manipulating the keto-enol tautomerism allows chemists to design molecules with desired reactivity and binding properties. As we advance in drug design, a comprehensive understanding of these tautomeric systems is crucial for developing new therapeutic agents that can achieve specific biological activities. This understanding elevates **organic synthesis** within pharmaceutical research and application.
Recent Advances and Future Research Directions
The field of keto enol tautomerism continues to face exciting developments driven by ongoing research. Studies leveraging advanced techniques like *molecular dynamics* and *crystallographic studies* have provided deeper insights into tautomer stability and dynamics. Future research might focus on exploring the influence of electronic effects and substituent variability on tautomerization, further refining methods for evaluating and predicting reaction pathways. With innovations in synthetic methodologies, the scope for understanding keto enol tautomerism will expand significantly within emerging fields in chemistry.
Key Takeaways
- Keto enol tautomerism entails the dynamic equilibrium between keto and enol forms in organic compounds, influenced by several factors, including solvent effects and substituents.
- Understanding the mechanisms of proton transfer and reaction pathways leads to enhanced predictions of reactivity and synthetic applications in organic chemistry.
- The significance of keto-enol tautomerism is particularly evident in applications relating to drug design and medicinal chemistry, where manipulating tautomer forms can optimize efficacy.
- Future research areas promise deeper insights into electronic effects, stability determinations, and the roles of solvents in influencing tautomer equilibria.
FAQ
1. What is keto enol tautomerism?
Keto enol tautomerism is a chemical phenomenon where two structural isomers—the keto form with a carbonyl group and the enol form with a hydroxyl group—interconvert through proton transfer mechanisms. This equilibrium is vital in organic chemistry, influencing stability, reactivity patterns, and reaction mechanisms across various compounds.
2. What factors influence the keto-enol equilibrium?
Factors that affect keto-enol equilibrium include solvent polarity, temperature, and the presence of substituents that can stabilize the tautomeric forms. Highly polar solvents can stabilize the enol form through solvation, while certain substituents may enhance the acidity of the keto form or promote enolization.
3. How does temperature impact tautomerism?
Temperature impacts tautomerism significantly by shifting the equilibrium between keto and enol forms. Generally, increased temperature favors tautomers that might be thermodynamically less stable, thereby influencing the outcomes of synthetic applications and the efficiency of reactions.
4. What are the practical applications of keto enol tautomerism?
Keto enol tautomerism has numerous practical applications, especially in fields like medicinal chemistry and organic synthesis. The reactive enol form often serves as a nucleophile in various reactions, aiding in the synthesis of complex compounds crucial for drug development and bioactive molecules.
5. How can researchers study keto enol tautomerism?
Researchers employ various techniques to study keto enol tautomerism, including spectroscopic methods like **NMR analysis** and **IR spectroscopy**, which provide insights into the structural characteristics of tautomers. Additionally, molecular dynamics simulations help elucidate the stability and dynamics of the tautomeric systems for comprehensive analysis.
6. Why is understanding tautomerism important in organic chemistry?
Understanding tautomerism is vital in organic chemistry as it offers fundamental insights into isomerism, reaction mechanisms, and the properties of organic compounds. It equips chemists with knowledge to predict how different conditions will favor one form over the other, thereby informing their choice of reactants and reaction conditions in synthesis.
7. What are some future research directions in keto enol tautomerism?
Future research directions in keto enol tautomerism may focus on exploring the electronic effects of different substituents on tautomer stability and exploring how advanced analytical techniques can bridge gaps in understanding reaction kinetics and mechanisms, providing deeper insights into the promotional pathways in organic reactions.